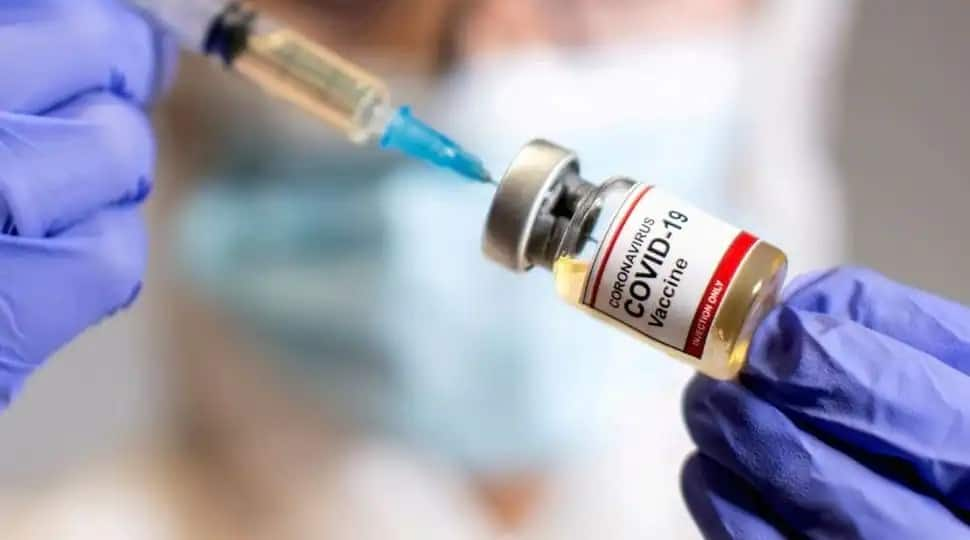
Hyderabad-based Biological E’s CORBEVAX has become the first vaccine in India to get DCGI approval for COVID-19 booster dose for adults who have received two doses of Covishield or Covaxin. According to the Statement released by Biological E, its vaccine Corbevax got DCGI approval for a heterologous COVID-19 booster shot. People who are 18 years & above and have already taken two doses of either COVISHIELD or COVAXIN can now receive a dose of CORBEVAX
The vaccine can be taken after 6 months of two doses of Covaxin or Covishield vaccines for restricted use in emergency situation, the company said. Mahima Datla, Managing Director, Biological E Limited, said, "We are very happy with this approval, which will address the need for Covid-19 booster doses in India. We have crossed yet another milestone in our Covid-19 vaccination journey. This approval reflects once again the sustained world class safety standards and high immunogenicity of CORBEVAX.”