Sputnik Light, the single-dose COVID vaccine, gets DCGI nod for phase 3 trials in India
The nod comes after a recent study published in the medical journal The Lancet said that Sputnik Light showed 78.6 to 83.7 per cent efficacy against COVID-19, significantly higher than most two-shot vaccines.
Sputnik V soft launch not put on hold, Dr Reddy's Laboratories issues statement
Dr Reddy's Laboratories said that the nationwide soft launch of Sputnik V has reached over 50 cities and towns across the country.
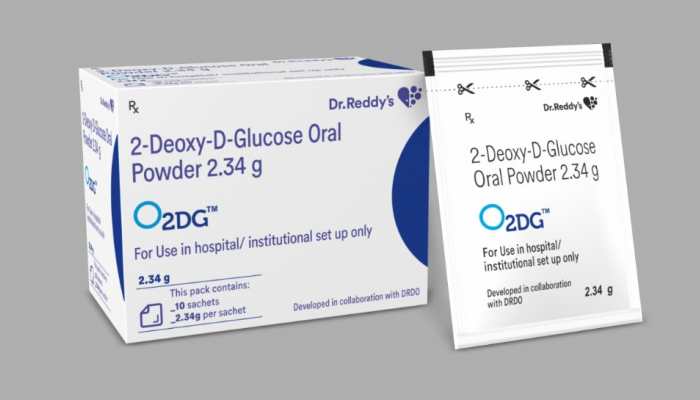
Jul 13, 2021, 09:51 AM ISTDr Reddy's Laboratories announces commercial launch of anti-COVID 2-DG drug
The Hyderabad based pharmaceutical company said that in the initial weeks, it will make the drug available in hospitals across metros and Tier 1 cities, and subsequently expand coverage to the rest of India.
Jun 28, 2021, 11:54 AM ISTBeware! 2DG COVID medicine will not see the daylight before June
The generic drugmaker released a statement on social media saying the anti-COVID remedy oral drug 2DG has not yet been launched into the market. Here, we advise our readers to remain vigilant and refrain themselves from falling into the trap of black marketers who lure them by offering the 2DG drug in exchange for a hefty amount. We request you to share this article with your friends and relative so as to alert your surroundings.
May 20, 2021, 21:01 PM IST
Good news! Phase 2 trial of this COVID-19 vaccine may resume in India soon
An expert panel at the Central Drugs Standard Control Organisation (CDSCO) recommended granting permission to the second phase clinical trial of Russia vaccine Sputnik V.
Oct 17, 2020, 10:59 AM ISTDr Reddy's shares slip nearly 3%; mcap drops by Rs 1,082 crore
In terms of equity volume, 1.57 lakh shares of the company were traded on BSE and over 17 lakh shares changed hands on NSE during the day.
Sep 08, 2017, 18:16 PM ISTDr Reddy's gets favourable verdict in patent litigation in US
Dr Reddy's stock was the biggest gainer among 30 Sensex scrips.
Sep 01, 2017, 16:06 PM ISTClass action lawsuit filed against Dr Reddy's in US
Drug firm Dr Reddy's Laboratories on Monday said a class action lawsuit has been filed against it in the US by an investor alleging misleading statements in violation of the US federal securities laws.
Aug 28, 2017, 15:28 PM ISTDRL says no claims against it for monetary damages in US patent case
There are no claims against drug major Dr Reddy's Laboratories informed the company for monetary damages informed the company through a BSE filing on Friday.
Feb 17, 2017, 14:32 PM IST7 FDI proposals worth Rs 100 crore get FIPB approval
Inter-ministerial body FIPB on Thursday cleared seven proposals envisaging foreign investment of Rs 100 crore.
Oct 27, 2016, 20:31 PM ISTUSFDA actions hurting exports, need govt intervention: Dr Reddy's
Asserting that regulatory action by the US Food and Drug Administration (USFDA) on leading Indian firms has impacted exports from the country, Dr Reddy's Laboratories Chairman Satish Reddy Wednesday asked for a dialogue between government and US health regulator.
Apr 06, 2016, 20:03 PM ISTDr Reddy's to invest in startups
Dr Reddy's Laboratories (DRL) will be investing in startups besides partnering with external entities for research and innovation.
Mar 07, 2016, 18:16 PM ISTDr Reddy's board approves share buyback for Rs 1,569.4 crore
Drug major Dr Reddy's will buyback around 44.85 lakh shares, accounting for about 2.6 percent of the existing paid up capital of the company, for up to Rs 1,569.4 crore.
Feb 17, 2016, 21:12 PM ISTDr Reddy's Q3 net profit rises marginally to Rs 579 crore
Dr Reddy's Laboratories today posted a marginal increase in consolidated net profit at Rs 579.2 crore for the third quarter ended December 31, hit by weak sales in the emerging markets like Russia, CIS nations and Romania.
Feb 09, 2016, 19:48 PM ISTTata Motors only Indian firm on top-50 global R&D list
Tata Motors has entered the top-50 league of the world's biggest companies in terms of their R&D investments, topped by German automaker Volskwagen.
Dec 20, 2015, 17:44 PM ISTUSFDA gives Dr Reddy's addl time to respond to warning letter
Dr Reddy's Laboratories Ltd (DRL) Thursday said the USFDA has extended the time-frame for replying to the warning letter issued to the company by about two weeks to December 7.
Nov 26, 2015, 18:38 PM ISTDr Reddy's shares slump 8.5%; m-cap dips Rs 4,746 crore
The stock fell on reports that the US Food and Drug Administration (USFDA) might withhold approval of the company's fresh drugs and stop import after it found several violations at three of its plants.
Nov 26, 2015, 18:28 PM ISTUS FDA warns of DRL drug import ban if flaws are not fixed
The US Food and Drug Administration (US FDA), which had issued a warning letter to Dr Reddy's Laboratories over quality issues, has said it might withhold approval of the company's fresh drugs and stop import if no corrective action is taken.
Nov 25, 2015, 18:34 PM ISTDr Reddy's accused of making false claims about financials, shares slump 7%
US-based law firm Lundin Law, which specialises in securities litigations is investigating claims against Dr Reddy's Laboratories for possible violations of federal security laws, which the drug maker has denied.
Nov 19, 2015, 14:30 PM ISTUS court asks Dr Reddy's Laboratories to halt generic sale of esomeprazole
A US court has directed Dr Reddy's Laboratories to temporarily stop selling its generic version of esomeprazole in the US market.
Nov 10, 2015, 17:41 PM IST