Maharashtra FDA Exposes McDonald's For Alleged Food Quality Concerns, Food Chain Responds
Maharashtra FDA Commissioner Abhimanyu Kale stated that they received customer feedback regarding the use of cheese analogues instead of real cheese.
Feb 23, 2024, 19:32 PM ISTSun Pharma Recalls Over 34k Bottles of Generic Drug in US due to Manufacturing Issues
The Mumbai-based drug major had produced the lot at its Halol-based manufacturing facility in Gujarat. The affected lot was later distributed in the market by its US-based unit.
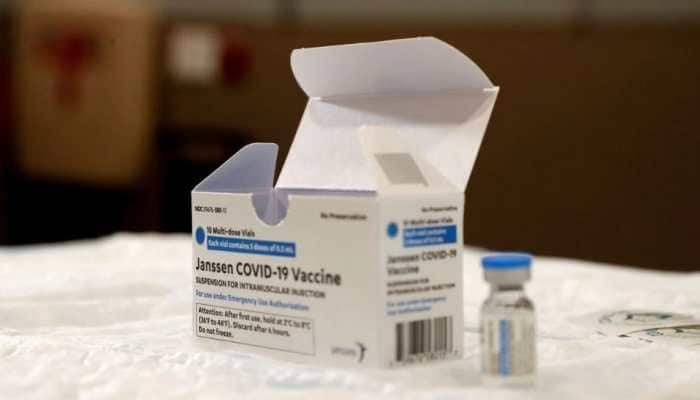
Feb 11, 2023, 13:53 PM ISTUS FDA limits use of Johnson & Johnson Covid-19 vaccine over blood clot risk
US regulators have strictly limited who can receive Johnson & Johnson's COVID-19 vaccine due to the ongoing risk of rare but serious blood clots.
May 06, 2022, 07:20 AM ISTInspectIR: FDA authorizes 1st breath test for Covid-19 infection in US
The test, which can provide results in less than three minutes, must be carried out under the supervision of a licensed health care provider.
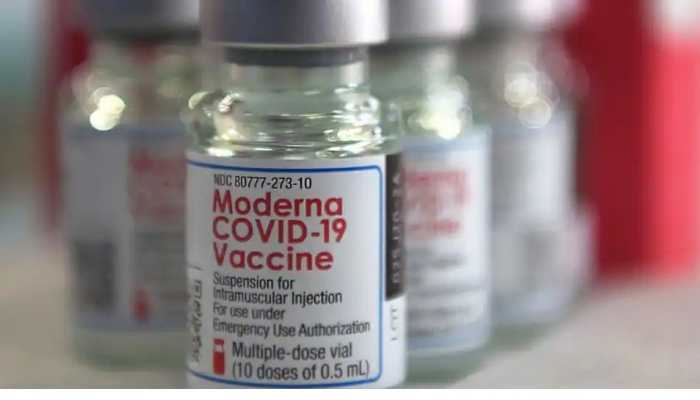
Apr 15, 2022, 06:38 AM ISTModerna seeks FDA approval for fourth Covid vaccine to be used as booster shot
Moderna is now seeking Food and Drug Administration (FDA) approval for its fourth shot of the Covid-19 vaccine to be administered as a booster dose in eligible adults.
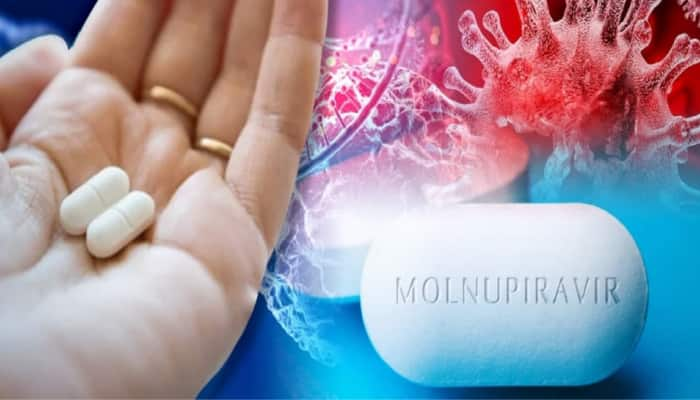
Mar 18, 2022, 09:55 AM ISTMolnupiravir benefits outweigh risk among Covid patients: Experts
An expert panel of the Central Drugs Standard Control Organisation had recently approved the antiviral Molnupiravir for restricted use in emergency situations, However, ICMR has raised concern over its side effects.
Jan 11, 2022, 10:44 AM ISTIn a first, US clears AstraZeneca antibody for long-term prevention against COVID-19 infection
The AstraZeneca antibody-drug cleared by the US Food and Drug Administration on Wednesday is different. It's the first authorised for long-term prevention against COVID-19 infection, rather than short-term treatment.
Dec 09, 2021, 07:52 AM ISTCovaxin for kids: Bharat Biotech's partner requests approval for ages 2-18 in US
Bharat Biotech's US partner Ocugen has approached US Food and Drug Administration (FDA) for Emergency Use Authorisation (EUA) of Covaxin for children below 18
Nov 06, 2021, 11:23 AM ISTUS backs Pfizer's low-dose COVID-19 vaccine for kids, FDA approval expected soon
A Food and Drug Administration advisory panel voted unanimously, with one abstention, that the vaccine's benefits in preventing COVID-19 in that age group outweigh any potential risks including a heart-related side effect that's been very rare in teens and young adults despite their use of a much higher shot dose.
Oct 27, 2021, 10:17 AM ISTUS approves new booster of COVID-19 vaccines, mix and match dose
“The use of a single booster dose of the Janssen COVID-19 vaccine may be administered at least 2 months after completion of the single-dose primary regimen to individuals 18 years of age and older," FDA said.
Oct 21, 2021, 06:30 AM ISTFDA approves booster shots of Pfizer COVID-19 vaccine for elderly, high-risk Americans
The booster dose is to be administered at least six months after completion of the second dose, and the authorization would include people most susceptible to severe disease and those in jobs that left them at risk, the FDA said.
Sep 23, 2021, 06:26 AM IST'Pfizer COVID vaccine may be authorised for children aged between 5 to 12 by October'
Currently of the three COVID-19 vaccines used in the US, only the Pfizer-BioNTech vaccine has been granted the emergency use authorisation for children aged 12 and older.
Aug 30, 2021, 13:45 PM ISTUS FDA okays arthritis drug for treatment of COVID-19
Actemra is a monoclonal antibody that reduces inflammation and is given by intravenous infusion that is FDA-approved for multiple inflammatory diseases, including rheumatoid arthritis.
Jun 27, 2021, 13:02 PM ISTFDA rejects emergency use authorisation for Covaxin in a setback for India-made jab
FDA suggests Biologics Licence Application - a "full approval" mechanism by the FDA for drugs and vaccines - route for approval in the United States
Jun 11, 2021, 11:50 AM ISTCOVID jabs for kids aged 2 to 11: Pfizer to seek approval in Sept
Pharmaceutical major Pfizer will likely seek the US Food and Drug Administration's emergency authorisation to administer its coronavirus vaccine among children between the ages of 2 and 11 in September, the media reported.
May 05, 2021, 16:08 PM ISTUS FDA to authorise Pfizer jabs for 12 to 15-year-old by next week
The US Food and Drug Administration (FDA) is planning to open Pfizer-BioNTech coronavirus vaccine for adolescents aged 12 to 15 years by early next week, according to federal officials, the media reported.
May 04, 2021, 15:19 PM ISTCOVID oral pill could be ready by year-end: Pfizer CEO
The drug is part of a class of medicines called protease inhibitors and works by inhibiting an enzyme that the virus needs to replicate in human cells.
May 03, 2021, 09:58 AM ISTUS authorizes Pfizer's COVID-19 vaccine, first shot to be administered in 'less than 24 hours'
The US Food and Drug Administration authorized Pfizer`s Covid-19 vaccine for emergency use on Friday for the prevention of coronavirus disease in individuals 16 years of age and older.
Dec 12, 2020, 09:48 AM IST3 former US presidents willing to take COVID-19 vaccine publicly to boost confidence
Health care workers and nursing home residents should be at the front of the line, according to the influential Advisory Committee on Immunization Practices. That encompasses about 24 million people out of a U.S. population of around 330 million.
Dec 04, 2020, 07:46 AM ISTUS regulator FDA allows emergency use of remdesivir drug for coronavirus COVID-19 patients
US regulators on Friday allowed emergency use of an experimental drug that appears to help some coronavirus patients recover faster.
May 02, 2020, 07:53 AM IST